Chemistry plays a crucial role in industries ranging from pharmaceuticals to food production, and one reaction that holds significant industrial importance is the hydrolysis of methyl formate (HCOOCH₃) with water (H₂O). This reaction is not just an academic concept—it has real-world applications in manufacturing, environmental science, and laboratory research. In this guide, we will explore what this reaction is, how it works, its uses, benefits, production process, and modern applications.
What is HCOOCH₃ + H₂O?
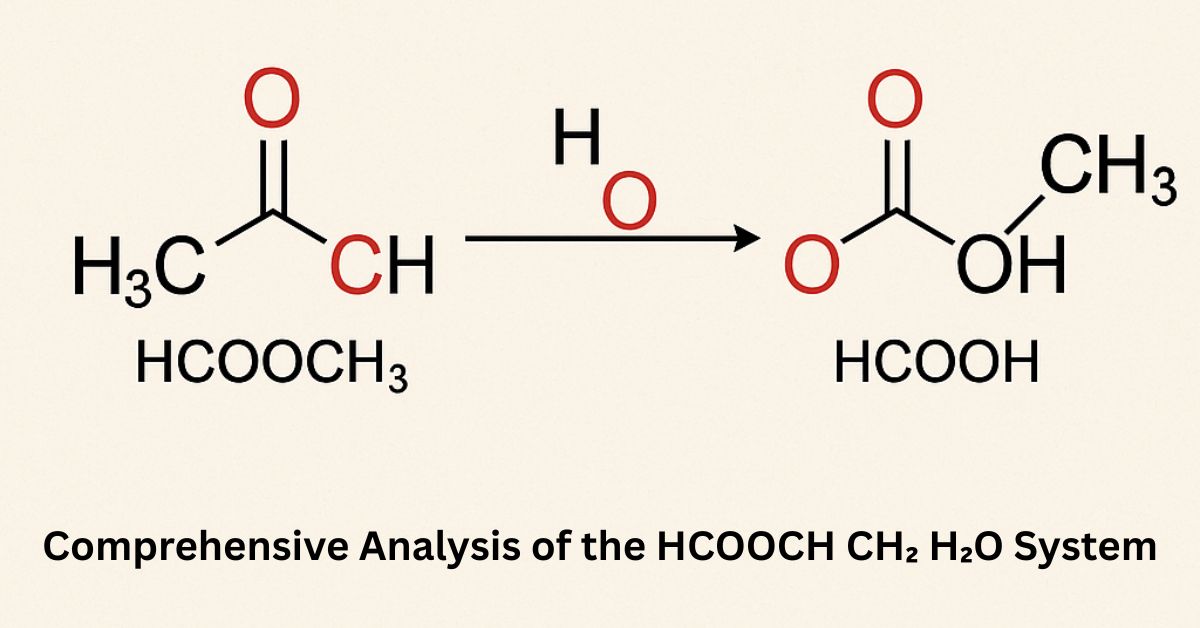
The chemical formula HCOOCH₃ represents methyl formate, an ester derived from formic acid (HCOOH) and methanol (CH₃OH). When methyl formate reacts with water (H₂O), the process is known as hydrolysis. This reaction breaks the ester bond, producing methanol (CH₃OH) and formic acid (HCOOH).
Chemical equation:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
This reaction is an example of an ester hydrolysis, often catalyzed by acids or bases to increase the reaction rate.
How Does Methyl Formate Hydrolysis Work?
The hydrolysis reaction involves breaking the ester bond between the methyl group (CH₃) and the formate group (HCOO). Water acts as a nucleophile, attacking the carbonyl carbon of methyl formate. This leads to the formation of a tetrahedral intermediate, which then breaks down into methanol and formic acid.
There are two main types of hydrolysis:
- Acid-catalyzed hydrolysis – Uses an acid like sulfuric acid as a catalyst.
- Base-catalyzed hydrolysis (saponification) – Uses a strong base like sodium hydroxide.
Acid-catalyzed hydrolysis is more common in industrial applications because it provides better control over reaction conditions.
Why is This Reaction Important?
The hydrolysis of methyl formate is not just a textbook reaction—it has valuable applications in chemical manufacturing. The products, methanol and formic acid, are both important industrial chemicals:
- Methanol is used as a fuel, solvent, and precursor to various chemicals.
- Formic acid is used in leather processing, textile finishing, rubber production, and as a preservative.
Because the reaction uses relatively inexpensive raw materials, it is an efficient and cost-effective method for producing these chemicals.
Industrial Uses of HCOOCH₃ + H₂O Reaction
This hydrolysis reaction finds applications in multiple industries:
1. Chemical Manufacturing
Formic acid produced through hydrolysis is widely used as a feedstock for other chemicals, such as formate salts and esters. Methanol serves as a starting material for formaldehyde, acetic acid, and biodiesel.
2. Textile and Leather Industry
Formic acid is essential for tanning leather and finishing fabrics. Hydrolysis of methyl formate ensures a consistent supply of high-purity acid for these purposes.
3. Rubber Industry
Formic acid helps in coagulating latex rubber, making this reaction important for rubber processing plants.
4. Pharmaceuticals
Methanol and formic acid derivatives are used in the synthesis of several pharmaceutical intermediates.
Benefits of the Hydrolysis Process
The HCOOCH₃ + H₂O reaction offers several advantages:
- High yield – Produces methanol and formic acid efficiently.
- Cost-effectiveness – Uses readily available raw materials.
- Scalability – Suitable for both laboratory and industrial-scale production.
- Versatility – Reaction can be adjusted for acid or base catalysis.
- Eco-friendliness – Compared to some chemical processes, hydrolysis generates fewer harmful byproducts.
Step-by-Step Production Process
The industrial hydrolysis of methyl formate involves several controlled steps:
- Raw Material Preparation
Methyl formate is typically produced by reacting methanol with carbon monoxide. This ester is then purified before hydrolysis. - Catalyst Selection
Acid catalysts (such as H₂SO₄) or base catalysts (such as NaOH) are chosen based on desired reaction conditions. - Reaction Stage
Methyl formate is mixed with water in a reactor, and the catalyst is added. The mixture is heated to accelerate hydrolysis. - Separation
After completion, methanol and formic acid are separated using distillation. - Purification
Both products are further refined to meet industry-grade purity standards.
Modern Applications and Innovations
Advancements in chemical engineering have improved the efficiency of methyl formate hydrolysis:
- Continuous flow reactors for faster production.
- Green chemistry methods that use less hazardous catalysts.
- Renewable methanol from biomass sources combined with CO₂ capture.
- On-site formic acid production in industries to reduce transportation costs.
These developments are making the reaction more sustainable and aligned with environmental goals.
Safety Considerations
While the reaction is relatively straightforward, both methyl formate and its products require careful handling:
- Methyl formate is flammable and toxic if inhaled in high concentrations.
- Formic acid is corrosive and can cause burns.
- Proper ventilation, protective clothing, and adherence to chemical safety protocols are essential.
Environmental Impact
The hydrolysis of methyl formate is generally considered an environmentally friendly process compared to other chemical syntheses. However, waste management and emission control remain critical. Modern facilities use closed-loop systems to minimize environmental release of volatile organic compounds (VOCs).

FAQs About HCOOCH₃ + H₂O
1. What is the reaction between HCOOCH₃ and H₂O?
It is the hydrolysis of methyl formate, producing formic acid and methanol.
2. Is the reaction reversible?
Yes, under certain conditions, the reverse reaction—esterification—can occur, forming methyl formate from methanol and formic acid.
3. Why is acid catalysis preferred in industry?
Acid catalysis provides better control, higher yields, and fewer unwanted side reactions compared to base-catalyzed hydrolysis.
4. Where is formic acid used?
In leather tanning, textile finishing, rubber processing, food preservation, and as a chemical intermediate.
5. Can this reaction be done in a lab setting?
Yes, but it requires proper safety measures due to the toxicity and flammability of methyl formate.